Unexpected Early Response in Oral Bioavailability of Ascorbic Acid
Vitamin C Blood Levels can be Higher from Oral Intake than Intravenous Infusion in the Early Minutes
© Owen Fonorow and Steve Hickey
INTRODUCTION
Repeatable and inexpensive experiments have cast doubt on the hypothesis that only 200 mg of vitamin C taken orally can be absorbed. We measured vitamin C blood plasma levels every minute for the first 40 minutes using a novel approach. By contrast to the prevailing paradigm, our results suggest that up to 4,000 mg of ascorbic acid taken by mouth can produce the same rapid increase in plasma concentration as an intravenous infusion. Previous studies did not sample blood levels during this early stage of oral intake. We confirmed that specific glucose meters do provide a reproducible measure of ascorbate (Vitamin C) concentration. Researchers had previously demonstrated the ability of specific glucose meters to measure high levels of ascorbate during and after Intravenous Vitamin C infusions (IV/C) [1](Ma, Sullivan, Schrick, Choi, He, Lierman, Chen, 2013). Our initial aim was to confirm the adequacy of the method. However, we observed that the meters are responsive to lower oral intakes, and discovered an initial high rate of oral absorption, which may add to understanding of the pharmacokinetics of ascorbate and have clinical and nutritional implications.
METHODS
In 2012, we observed that the Abbott Laboratories FreeStyle® Lite glucose meter was responding quantitatively to vitamin C in blood plasma. This is consistent with the similarity of glucose and ascorbate molecules. Biologically, this similarity leads to the cellular uptake of dehydroascorbate by GLUT [2, 3, 4](Bürzle & Hediger, 2012), (Corti, Casini, Pompella, 2010), (Szarka & L?rincz, 2013). Hence, the sensitivity to ascorbate in glucose meters is expected. The potential “error” due to high vitamin C is mentioned in the glucose meter product’s user manuals. Initially, the quantitative response was based on direct observations and reports from members of the Vitamin C Foundation [5](Fonorow, 2015). The observations suggested that there is a practical and inexpensive way of estimating in vivo vitamin C levels.
Data was obtained using three Abbott FreeStyle® Lite glucose meters that were purchased locally between 2013 and 2015. Each Abbott device was checked to confirm that it responded linearly and proportionately to ascorbate concentrations in test solutions comparable with the concentrations expected while the subject was undergoing intravenous infusions of vitamin C. The data estimation was the result of three different meter readings alternated so that glucose meter A measured minute 1, and minute 4, etc. The mg/dl are the units that USA glucose meters report. These numbers are not correct ascorbate concentrations, but are used to show relative changes in blood ascorbate.
Plasma glucose levels were expected to be approximately constant over the period of data collection. The subject in these experiments was a 61 year-old male, insulin-dependent diabetic. These experiments were run at on the same individual, at the same time, early in the morning after fasting during sleep for at least 8 hours. The lack of endogenous insulin production in this individual minimized any physiological insulin related response in the control of glucose or ascorbate levels.
In our first experiment, we measured relative ascorbate levels during an intravenous vitamin C infusion directly into the blood stream. As is required for vitamin C intravenous infusions, the vitamin was administered as sodium ascorbate. Because the vitamin C is the ascorbate ion, we accounted for the sodium by adjusting the sodium ascorbate dosage to 11.3 grams. This adjustment ensured that 10 grams of vitamin C was endogenously introduced to the subject, allowing comparison between all experiments.. Figure 1 plots the baseline data from all three meters during the sodium ascorbate IV/C infusion.
Experiment 2 measured the relative ascorbate levels while oral vitamin C (as ascorbic acid) was introduced at the same rate (250 mg/minute) as the sodium ascorbate intravenous infusion. Plasma levels were again measured minute-by-minute, alternating the meters, over the same duration as the short IV/C. Figure 2 compares the plasma response of the slow introduction of oral vitamin C as ascorbic acid with the data from the first experiment, sodium ascorbate IV infusion. The data from all three meters are averaged in the second plot. The baseline IV/C sodium ascorbate data are averaged.
Experiment 3 measured the relative ascorbate concentrations after a single, large 10 gram oral dose of ascorbic acid. The 10 grams is the equivalent vitamin C to the amount given by intravenous infusion in the first experiment. Measurements were minute-by-minute over the same 40 minute period as the baseline IV/C. Figure 3 compares the plasma response of the single oral dosage of vitamin C as ascorbic acid with the data from the first experiment, sodium ascorbate IV/C infusion. The data from all three meters is plotted to show the rapid changes in blood levels from the ascorbic acid gulp. The IV/C baseline data are averaged.
Experiment 4 measured the relative ascorbate concentrations after a single, large, 11.3 gram oral dose of sodium ascorbate. This dosage provided the equivalent 10 grams of vitamin C. Measurements were minute-by-minute, alternating meters, over the same 40 minute period as the baseline IV/C. Figure 4 compares the plasma response of a large dose of oral sodium ascorbate with the equivalent large oral dose of ascorbic acid. The data from all three meters are averaged.
The amount of vitamin C administered in all the experiments was the same (10 grams of ascorbate.)
Experiment 5 measured a 10 gram single oral dose of glucose for comparison against the oral ascorbic acid gulp.
RESULTS
Experiment 1 – 10 gram IV/C infusion (11.3 gram sodium ascorbate)
The first experiment measured “glucose” levels during an intravenous vitamin C infusion, where no glucose was present.
The chart in Figure 1 shows the steady rising blood levels, until the IV/C bag emptied and the relative blood levels then began to decline. Three Abbott meters were used to minimize a random measurement error in one meter.
We performed a regression analysis using Microsoft Excel 2007 Data Analysis package on the meters which provided significant support for the good agreement on visual inspection of the measurements. The multiple R value for meters A-IV and B-IV was 0.82 (F=30.69; P=5.66×10-5). With A-IV and C-IV the R was 0.72 (F=14.89; p=1.74×10-3). For B-IV and C-IV the R was 0.91 (F=66.58; p=1.09×10-6).
The consistency among these measurements suggest that these meters measured vitamin C blood levels. However, these measurements are mg/dl in terms of glucose, and are not accurate vitamin C concentrations.
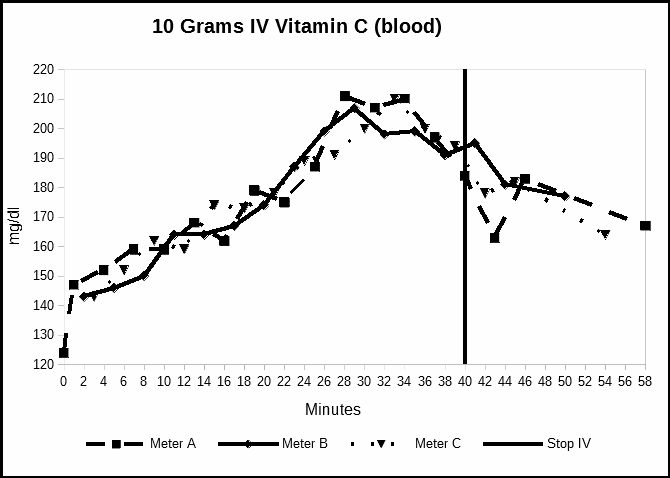
Fig 1. Measurements at one-minute intervals during a 10 gram intravenous infusion of vitamin C.
The IV bag emptied at minute 34, and the line drained and the needle was removed at minute 40. The increase in concentration from the IV is also consistent with our in vitro measurements. The decline at the end of the IV illustrates the short 30-minute half-life of the vitamin. To a first approximation, we take the fasting subject’s glucose levels to be constant over this period.
Experiment 2 – Oral Ascorbic Acid at the Same Rate as IV
The second experiment introduced vitamin C orally at the same rate (250 mg/minute) as the intravenous infusion in Experiment 1. There was little difference in Vitamin C blood levels between oral ascorbic acid and intravenous sodium ascorbate for the first 15-16 minutes (4,000 mg). After this period, concentrations from oral intake dropped off. This experiment shows that 4 grams of oral vitamin C as ascorbic acid enters the blood stream as well as vitamin C introduced directly by vein. Indeed, the initial oral measurements appear slightly greater than were obtained with the IV/C suggesting an efficient absorption through the stomach wall.
The subject was administered vitamin C at the same rate as the infusion, i.e., 250 mg of ascorbic acid by mouth every minute for 40 minutes.
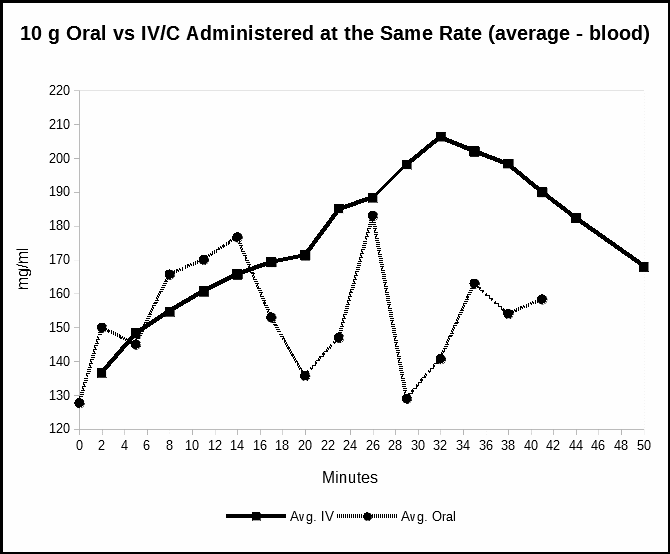
Figure 2 compares the response from oral ascorbic acid when given to match the rate of the intravenous infusion.
The results of the experiment show comparable blood levels between oral and IV/C for the first fifteen minutes. After this period, the oral blood levels declined, relative to the IV/C. The decline in the rate of absorption may reflect the increase in stomach pH as ascorbic acid buffered the stomach contents (Hcl).
Experiment 3 – 10 gram single oral dose of Ascorbic Acid compared to IV
The third experiment measured vitamin C after a single large (10 gram) dose of ascorbic acid taken by mouth. The data showed that the blood levels spiked as early as minute 3 to levels higher than the intravenous infusion achieved over 40 minutes. The spiking blood level event was over by minute 12. Anecdotally these totally unexpected results suggest that oral ascorbic acid can produce transient high blood levels of vitamin C. These transient high levels would be missed by any experiment with a longer measurement period. Rapid minute-by-minute measurements are required to observe these high values.
In Figure 3, we measured the blood concentrations after a single oral dose of 10 grams of ascorbic acid.
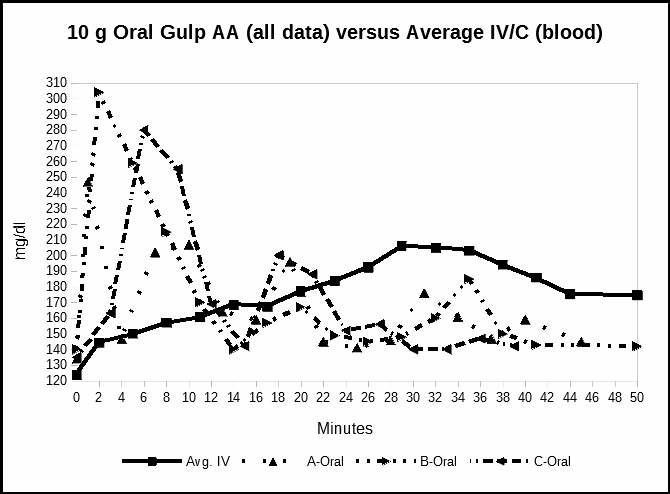
Fig. 3. Time series following a single oral dose versus IV – mg/dl versus time in minutes. All three meters are plotted.
Unexpectedly, blood levels of ascorbic acid spiked early and profoundly. The maximum levels appeared as early as minute 3, and varied slightly from minute 3 to minute 7. The initial peak had declined back towards baseline IV/C by the 15th minute. This initial high level was consistent with the results in Experiment 2 in suggesting a rapid initial absorption of oral ascorbic acid.
We investigated whether the quick entry into the blood stream was via the mucous membranes in the mouth or the stomach. We did not find any significant blood “glucose” elevation holding the 10 gram vitamin C solution in the mouth for a long period..
Our original finding is that during the first 12 minutes after a single large dose of ascorbic acid, blood concentrations of vitamin C were substantially higher than the levels produced by the intravenous infusion. The entry into the blood during the first few minutes is most likely from the vitamin’s passage through the stomach lining.
Experiment 4 – Single 11.3 gram oral dose of Sodium Ascorbate
The fourth experiment measured vitamin C after a single large (11.3 gram) dose of sodium ascorbate taken by mouth.
In Figure 4, we compare the blood concentrations after a single oral dose of 10 grams of Vitamin C as sodium ascorbate with the single 10 gram dose of ascorbic acid data from experiment 3.
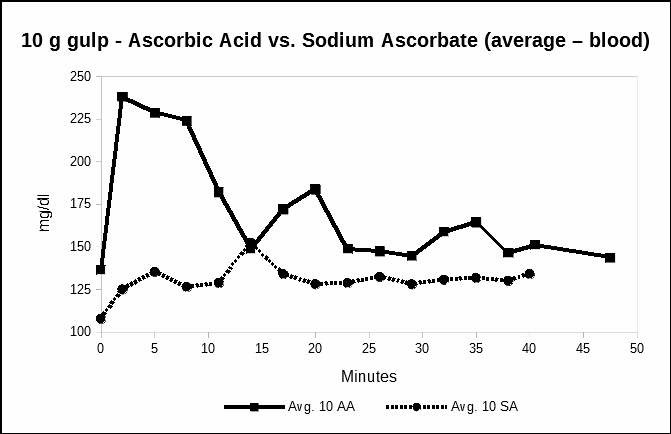
Fig. 4. Time series following a single oral dose of 11.3 grams sodium ascorbate compared with 10 grams ascorbic acid Date from all three meters are averaged.
Figure 4 illustrates the difference in relative vitamin C blood levels depending on whether the vitamin is taken as ascorbic acid, or sodium ascorbate. The data showed a sodium ascorbate produces a slower rise in vitamin C blood levels. No high-level transient spikes were measured.
This experiment with oral sodium ascorbate suggests that the form of the vitamin C may determine the rate at which vitamin C enters the blood stream. The blood concentration pattern after oral sodium ascorbate is markedly different from the oral ascorbic acid. Sodium ascorbate concentrations were lower, perhaps because sodium ascorbate requires more time for absorption into the blood stream as the vitamin travels past the stomach to the intestines.
Our calibration measurements hinted that the glucose meters may report the same sodium ascorbate and ascorbic acid concentrations differently. (These calibrations were in water, not blood, making the glucose meter readings difficult and prone to error.) Even if the blood levels are not directly comparable, the pattern of entry into the blood is markedly different between the two forms of vitamin C. The mildly acidic ascorbic acid has a rapid entry effect in the blood, while the rate of entry of the alkaline sodium ascorbate is slower and more like timed release..
Experiment 5 – 10 gram single oral dose of glucose
There was concern that somehow we were measuring glucose. As a control, in Figure 5, we measured the blood concentrations after a single oral dose of 10 grams of glucose. The fifth experiment repeated the method after a single large (10 gram) dose of glucose taken by mouth.
Figure 5 compares the oral ascorbic acid data from experiment 3 with the glucose experiment.
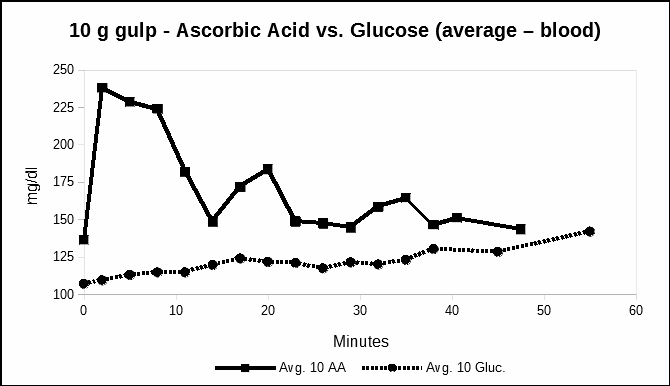
Fig. 5. Time series following a single oral dose of 10.0 grams glucose compared with 10.0 grams of ascorbic acid– mg/dl versus time in minutes. The average of all three meters are plotted.
In Figure 5, the blood levels of glucose were compared with vitamin C as ascorbic acid. The measurement values are presumably accurate for glucose. While the actual vitamin C concentration is unknown, the pattern entering the blood stream is different.
DISCUSSION
The vitamin C absorption into the blood stream findings reported here are generally practical because some glucose meters are sensitive to and can be used to report ascorbate levels in the blood [1](Ma, Sullivan, Schrick, Choi, He, Lierman, Chen, 2013). This sensitivity appears to give a linear quantitative response and reproducible measurements. While plasma glucose is greater than ascorbate levels, in a fasting individual glucose levels are expected to be approximately constant over the periods considered. Insulin may affect the experiments as the vitamin C (or glucose) is made available to enter cells. In this diabetic subject, we had the opportunity to monitor blood levels without this confounding factor. We had previously determined that the meters response to ascorbate was greater than that to glucose, presumably related to a redox aspect of the measurement. These minute-by-minute measurements are new to the literature, partially because of the practical difficulty in collecting, and then storing blood for so many measurements, but perhaps reflecting the unexpected nature of the rapid oral response.
Not every brand or model of glucose meter is as sensitive to ascorbate, and the details of the mechanisms are commercially sensitive. We tested several meters that did not provide a robust measure of ascorbate concentration. It is possible that the FDA may consider a glucose meter that reacts to vitamin C flawed. For this reason, manufacturers may be forced to upgrade their meters, rendering future versions of the Abbott and other meters unusable for vitamin C measurements. Ideally, a low-cost finger-prick ascorbate (vitamin C) meter will be made available that makes accurate measurements. A new company in New Zealand recently announced that they are developing such a meter.
We calibrated the Abbott FreeStyle® Lite glucose meters against accurately measured solutions of ascorbic acid equivalent to concentrations in the blood over the range .5 to 1.5 mg/dl [2](Bürzle & Hediger, 2012). The meter provided a linearly proportional response to the ascorbate concentrations measured. Here we are concerned with rapid changes in blood plasma relative to baseline. These experiments compared relative blood concentrations between oral and intravenous, and different forms of vitamin C
In this preliminary report, we show that during a slow intravenous infusion of 10 grams of vitamin C, three separate meters report a steady increase in measured ascorbate, consistent with the increased concentration of vitamin C in the blood. At the end of the infusion all three meters showed the decrease in blood levels consistent with ascorbate’s 30-minute half-life and the Dynamic Flow theory of ascorbate [6, 7](Hickey & Roberts, 2004 & 2005).
Our IV/C infusion data from vitamin C introduced directly by vein into the blood stream (experiment 1) provided an approximation to a 100% bioavailability. Experiment 2 compared oral and intravenous vitamin C introduced at the same rate. We expected that less vitamin C would enter the blood stream from oral absorption.
The minute-by-minute readings comparing the IV infusion for the first 15 minutes (4000 mg) is unprecedented, and leads to the reasonable conclusion that a similar amount of vitamin C entered the blood stream. Some prior research had reported that only about 250 mg can be absorbed before tissue saturation [8, 9](Levine M, Conry-Cantilena, Wang, 1996), (Levine, Padayatty, Espey, 2011). However, measuring vitamin C in the urine or waiting too long to begin blood measurements would have missed the rapid absorptio..n.
In the third experiment, a single dose of 10 grams of vitamin C as ascorbic acid was consumed all at once. Minute-by-minute measurements were compared to the slow intravenous infusion. The surprising finding is that in the first few minutes, the rapid oral absorption of 10 grams of ascorbic acid created higher blood levels than the low dose IV/C.
The measurements for ascorbic acid were unexpected. The blood concentrations between the baseline and oral ascorbic acid at the same rate were comparable and showed equivalent bioavailability to 4000 mg. The bioavailability of a large one-time dosage produced blood levels higher than the intravenous infusion of the same amount.
In the fourth experiment, a single dose of 11.3 grams of vitamin C as sodium ascorbate was consumed all at one time. These measurements were compared to oral ascorbic acid. Another (unanticipated) finding was the unexpected difference between the rates of absorption of the different forms of vitamin C.
While the ascorbic acid response was unexpected, it is consistent with known pharmacokinetics of weak acids. A weak acid like ascorbic acid is in the associated relatively non-polar state in the low pH of the stomach becoming more lipid soluble. Weak acids often absorb rapidly from the stomach. However, if the stomach acid is decreased the weak acid disassociates and the polarity inhibits transfer across cell membranes. The ascorbic acid would buffer the stomach pH (from pH ~1 to pH ~4) and inhibit its own absorption. Sodium ascorbate would be a more effective buffering agent and this would explain why the initial absorption spike was not observed.
One objection to these case study data is individual variation in this preliminary study. This reservation is accepted and is common to case studies, which nevertheless can convey interesting observational data. The measurement is direct, using a technique established elsewhere [1](Ma, Sullivan, Schrick, Choi, He, Lierman, Chen, 2013). Moreover, it would appear that some individual’s large intakes of vitamin C might be rapidly absorbed initially from the oral route. It remains to be established how frequent this phenomenon is in the population. Moreover, if replicated the observations may provide an alternative to IV administration of ascorbate now being clinically trialed in cancer [10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21](Chen, Stone, Sullivan, Drisko, Chen, 2011), Monti, Mitchell, Bazzan, Littman, Zabrecky, Yeo, Levine, 2012), (Wang, Yin, Wang, 2016), (Leekha, Gurjar, Tyagi, Rizvi, Verma, 2016), (Baek, Cho, Kim, Kim, Jung, 2017), (Campbell, Vissers, Wohlrab, Hicks, Strother, Bozonet, et al., 2016), (Frajese, Benvenuto, Fantini, Ambrosin, Sacchetti, Masuelli, Giganti, Modesti, Bei, 2016), (Aguilera, Muñoz-Sagastibelza, Torrejón, Borrero-Palacios, del Puerto-Nevado, Martínez-Useros, García-Foncillas, 2016), (Jung, Lee, Moon, Hong, Shin, Hwang, et al., 2016), (Chung, Kim, Ahn, Choi, Kim, Son, 2016), (Zhao, Wang, Song, Jin, Zhang, Gan, Yang, 2017), (Parro, Leshin, Levine, 2013). If the process we observed is one of rapid weak acid absorption from the stomach it may be possible to maintain stomach acidity and promote rapid oral absorption of large doses of vitamin C, at least in some individuals. Consistent with this interpretation we have observed that while ascorbic acid can be rapidly absorbed in to the blood stream, sodium ascorbate raises blood levels more slowly.
Vitamin C blood levels must be measured repeatedly within 30 to 40 minutes to obtain an accurate reading of how much vitamin C enters the blood stream. Cathcart described how people who are sick and under stress can tolerate very high oral intakes of vitamin C [22](Cathcart, 1981). The Cathcart bowel tolerance amounts, sometimes as high as 200 grams daily, are difficult to reconcile with the current paradigm if blood plasma saturates at 250 mg. Cathcart also reported that he could only obtain “a clinical ascorbate effect” orally with ascorbic acid, not mineral ascorbates. We might speculate that an increased stomach acidity in the sick can at least in part explain Cathcart’s observations.
The rapid early absorption and utilization of ascorbic acid presented here, previously unknown, may help explain what Cathcart reported.
ACKNOWLEDGEMENTS
Steven Hickey, PhD, whose advice and counsel was invaluable to this paper.
Thomas Hesselink, MD, who measured the ascorbate and oversaw the sodium ascorbate IV/C.
Forum member johnwen, who created the initial charts.
Vitamin C Foundation staff member Jerry Nowlin, who updated the charts.
Feedback from members of the Vitamin C Foundation forum.
REFERENCES
Aguilera, O., Muñoz-Sagastibelza, M., Torrejón, B., Borrero-Palacios, A., del Puerto-Nevado, L., Martínez-Useros, J., García-Foncillas, J. (2016). Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget, 7(30), 47954–47965. http://doi.org/10.18632/oncotarget.10087
Baek MW, Cho HS, Kim SH, Kim WJ, Jung JY. (2017). Ascorbic Acid Induces Necrosis in Human Laryngeal Squamous Cell Carcinoma via ROS, PKC, and Calcium Signaling. J Cell Physiol, 232(2):417-425. doi: 10.1002/jcp.25438.
https://www.ncbi.nlm.nih.gov/pubmed/27211910[14] Ascorbic Acid Induces Necrosis in Human Laryngeal Squamous Cell Carcinoma via ROS, PKC, and Calcium Signaling. Baek MW, Cho HS, Kim SH, Kim WJ, Jung JY. J Cell Physiol. 2017 Feb;232(2):417-425. doi: 10.1002/jcp.25438. PMID: 27211910
Bürzle M, Hediger MA. (2012). Functional and physiological role of vitamin C
transporters. Curr Top Membr, 70:357-75.
Campbell EJ, Vissers MC, Wohlrab C, Hicks KO, Strother RM, Bozonet SM, et al. (2016). Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic Biol Med, 99:451–62.10.1016/j.freeradbiomed.2016.08.027
Cathcart R.F. (1981). Vitamin, C.; titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med. Hypotheses, 7:1359–1376. doi: 10.1016/0306-9877(81)90126-2.https://www.ncbi.nlm.nih.gov/pubmed/7321921
Chen P., Stone J., Sullivan G., Drisko J. A., Chen Q. (2011). Anti-cancer effect of pharmacologic ascorbate and its interaction with supplementary parenteral glutathione in preclinical cancer models. Free Radical Biology and Medicine, 51(3):681–687. doi: 10.1016/j.freeradbiomed.2011.05.031.[15] Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Campbell EJ, Vissers MC, Wohlrab C, Hicks KO, Strother RM, Bozonet SM, Robinson BA, Dachs GU. Free Radic Biol Med. 2016 Oct;99:451-462. doi: 10.1016/j.freeradbiomed.2016.08.027. PMID: 27567539 https://www.ncbi.nlm.nih.gov/pubmed/27567539
Chung MK, Kim do H, Ahn YC, Choi JY, Kim EH, Son YI. (2016). Randomized Trial of Vitamin C/E Complex for Prevention of Radiation-Induced Xerostomia in Patients with Head and Neck Cancer. Otolaryngol Head Neck Surg, 155(3):423-30. doi: 10.1177/0194599816642418.
Corti A., Casini A. F., Pompella A. (2010). Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Archives of Biochemistry and Biophysics, 500(2):107–115. doi: 10.1016/j.abb.2010.05.014.
Fonorow, O. (2015, June 15). Bioavailability of Vitamin C. Retrieved from http://vitaminc.foundation/forum/viewtopic.php?f=3&t=11944
Frajese GV, Benvenuto M, Fantini M, Ambrosin E, Sacchetti P, Masuelli L, Giganti MG, Modesti A, Bei R. (2016). Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines in vitro. Oncology Letters, 11(6), 4224–4234. http://doi.org/10.3892/ol.2016.4506[16] Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines in vitro. Frajese GV, Benvenuto M, Fantini M, Ambrosin E, Sacchetti P, Masuelli L, Giganti MG, Modesti A, Bei R. Oncol Lett. 2016 Jun;11(6):4224-4234. PMID: 27313770 https://www.ncbi.nlm.nih.gov/pubmed/27313770
Hickey, S., & Roberts, H. (2004). Ascorbate: The science of vitamin C. Morrisville, NC: Lulu. https://www.amazon.com/s/ref=dp_byline_sr_book_1?ie=UTF8&text=Steve+Hickey&search-alias=books&field-author=Steve+Hickey&sort=relevancerankhttps://www.amazon.com/s/ref=dp_byline_sr_book_2?ie=UTF8&text=Hilary+Roberts&search-alias=books&field-author=Hilary+Roberts&sort=relevancerank
Hickey, S., & Roberts, H. (2005). Ridiculous Dietary Allowance. Lulu.com. Aguilera O, Muñoz-Sagastibelza M, Torrejón B, Borrero-Palacios A, Del Puerto-Nevado L, Martínez-Useros J, Rodriguez-Remirez M, Zazo S, García E, Fraga M, Rojo F, García-Foncillas J.Oncotarget. 2016 Jul 26;7(30):47954-47965. doi: 10.18632/oncotarget.10087.PMID: 27323830 https://www.ncbi.nlm.nih.gov/pubmed/27323830 [17] Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer.
Jung S-A, Lee D-H, Moon J-H, Hong S-W, Shin J-S, Hwang IY, et al. (2016). L-Ascorbic acid can abrogate SVCT2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free Radic Biol Med, 95:200–8.10.1016/j.freeradbiomed.2016.03.009
L-Ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells.[18] L-Ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Jung SA, Lee DH, Moon JH, Hong SW, Shin JS, Hwang IY, Shin YJ, Kim JH, Gong EY, Kim SM, Lee EY, Lee S, Kim JE, Kim KP, Hong YS, Lee JS, Jin DH, Kim T, Lee WJ. Free Radic Biol Med. 2016 Jun;95:200-8. doi: 10.1016/j.freeradbiomed.2016.03.009.PMID: 27012422 https://www.ncbi.nlm.nih.gov/pubmed/27012422[19] Randomized Trial of Vitamin C/E Complex for Prevention of Radiation-Induced Xerostomia in Patients with Head and Neck Cancer. Chung MK, Kim do H, Ahn YC, Choi JY, Kim EH, Son YI. Otolaryngol Head Neck Surg. 2016 Sep;155(3):423-30. Doi: 10.1177/0194599816642418. PMID: 27048670 https://www.ncbi.nlm.nih.gov/pubmed/27048670
Leekha A, Gurjar BS, Tyagi A, Rizvi MA, Verma AK. (2016). Vitamin C in synergism with cisplatin induces cell death in cervical cancer cells through altered redox cycling and p53 upregulation. J Cancer Res Clin Oncol, 142(12):2503-2514.
Levine M., Conry-Cantilena C., Wang Y. (1996). Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci, 93(8):3704–3709.
Levine M, Padayatty SJ, Espey MG. (2011). Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr, 2:78–88.
Ma, Y., Sullivan, G. G., Schrick, E., Choi, I.-Y., He, Z., Lierman, J., … Chen, Q. (2013). A Convenient Method for Measuring Blood Ascorbate Concentrations in Patients Receiving High-Dose Intravenous Ascorbate. Journal of the American College of Nutrition, 32(3), 187–193. http://doi.org/10.1080/07315724.2013.791167
Monti, D. A., Mitchell, E., Bazzan, A. J., Littman, S., Zabrecky, G., Yeo, C. J., … Levine, M. (2012). Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE, 7(1), e29794. http://doi.org/10.1371/journal.pone.0029794
Parrow, N. L., Leshin, J. A., & Levine, M. (2013). Parenteral Ascorbate As a Cancer Therapeutic: A Reassessment Based on Pharmacokinetics. Antioxidants & Redox Signaling, 19(17), 2141–2156. http://doi.org/10.1089/ars.2013.5372
Szarka A, L?rincz T. (2013). Cellular and intracellular transport of vitamin C. The physiologic aspects. Orv Heti, 154(42):1651-6. doi: 10.1556/OH.2013.29712.[20] Synergistic Apoptotic Effect of D-Fraction From Grifola frondosa and Vitamin C on Hepatocellular Carcinoma SMMC-7721 Cells. Zhao F, Wang YF, Song L, Jin JX, Zhang YQ, Gan HY, Yang KH. Integr Cancer Ther. 2016 May 5. pii: 1534735416644674. [Epub ahead of print]PMID: 27151580 https://www.ncbi.nlm.nih.gov/pubmed/27151580[21] Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Parrow NL, Leshin JA, Levine M. Antioxid Redox Signal. 2013 Dec 10;19(17):2141-56. doi: 10.1089/ars.2013.5372. Review. PMID: 23621620 https://www.ncbi.nlm.nih.gov/pubmed/23621620
Wang G, Yin T, Wang Y. (2016). In vitro and in vivo assessment of high-dose vitamin C against murine tumors. Exp Ther Med, (12):3058–3062.
Zhao F, Wang YF, Song L, Jin JX, Zhang YQ, Gan HY, Yang KH. (2017). Synergistic Apoptotic Effect of D-Fraction From Grifola frondosa and Vitamin C on Hepatocellular Carcinoma SMMC-7721 Cells. Integr Cancer Ther, 16(2):205-214. doi: 10.1177/1534735416644674.
1. Ma, Y., Sullivan, G. G., Schrick, E., Choi, I.-Y., He, Z., Lierman, J., … Chen, Q. (2013). A Convenient Method for Measuring Blood Ascorbate Concentrations in Patients Receiving High-Dose Intravenous Ascorbate. Journal of the American College of Nutrition, 32(3), 187–193. http://doi.org/10.1080/07315724.2013.791167
2. Bürzle M, Hediger MA. (2012). Functional and physiological role of vitamin C
transporters. Curr Top Membr, 70:357-75.
3. Corti A., Casini A. F., Pompella A. (2010). Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Archives of Biochemistry and Biophysics, 500(2):107–115. doi: 10.1016/j.abb.2010.05.014.
4. Szarka A, L?rincz T. (2013). Cellular and intracellular transport of vitamin C. The physiologic aspects. Orv Heti, 154(42):1651-6. doi: 10.1556/OH.2013.29712.
5. Fonorow, O. (2015, June 15). Bioavailability of Vitamin C. Retrieved from http://vitaminc.foundation/forum/viewtopic.php?f=3&t=11944
6. Hickey, S., & Roberts, H. (2004). Ascorbate: The science of vitamin C. Morrisville, NC: Lulu.
7. Hickey, S., & Roberts, H. (2005). Ridiculous Dietary Allowance. Lulu.com.
8. Levine M., Conry-Cantilena C., Wang Y. (1996). Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci, 93(8):3704–3709.
9. Levine M, Padayatty SJ, Espey MG. (2011). Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr, 2:78–88.
10. Chen P., Stone J., Sullivan G., Drisko J. A., Chen Q. (2011). Anti-cancer effect of pharmacologic ascorbate and its interaction with supplementary parenteral glutathione in preclinical cancer models. Free Radical Biology and Medicine, 51(3):681–687. doi: 10.1016/j.freeradbiomed.2011.05.031.
11. Monti, D. A., Mitchell, E., Bazzan, A. J., Littman, S., Zabrecky, G., Yeo, C. J., … Levine, M. (2012). Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE, 7(1), e29794. http://doi.org/10.1371/journal.pone.0029794
12. Wang G, Yin T, Wang Y. (2016). In vitro and in vivo assessment of high-dose vitamin C against murine tumors. Exp Ther Med, (12):3058–3062.
13. Leekha A, Gurjar BS, Tyagi A, Rizvi MA, Verma AK. (2016). Vitamin C in synergism with cisplatin induces cell death in cervical cancer cells through altered redox cycling and p53 upregulation. J Cancer Res Clin Oncol, 142(12):2503-2514.
14. Baek MW, Cho HS, Kim SH, Kim WJ, Jung JY. (2017). Ascorbic Acid Induces Necrosis in Human Laryngeal Squamous Cell Carcinoma via ROS, PKC, and Calcium Signaling. J Cell Physiol, 232(2):417-425. doi: 10.1002/jcp.25438.
15. Campbell EJ, Vissers MC, Wohlrab C, Hicks KO, Strother RM, Bozonet SM, et al. (2016). Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic Biol Med, 99:451–62.10.1016/j.freeradbiomed.2016.08.027
16. FRAJESE, G. V., BENVENUTO, M., FANTINI, M., AMBROSIN, E., SACCHETTI, P., MASUELLI, L., … BEI, R. (2016). Potassium increases the antitumor effects of ascorbic acid in breast cancer cell lines in vitro. Oncology Letters, 11(6), 4224–4234. http://doi.org/10.3892/ol.2016.4506
17. Aguilera, O., Muñoz-Sagastibelza, M., Torrejón, B., Borrero-Palacios, A., del Puerto-Nevado, L., Martínez-Useros, J., … García-Foncillas, J. (2016). Vitamin C uncouples the Warburg metabolic switch in KRAS mutant colon cancer. Oncotarget, 7(30), 47954–47965. http://doi.org/10.18632/oncotarget.10087
18. Jung S-A, Lee D-H, Moon J-H, Hong S-W, Shin J-S, Hwang IY, et al. (2016). L-Ascorbic acid can abrogate SVCT2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free Radic Biol Med, 95:200–8.10.1016/j.freeradbiomed.2016.03.009
19. Chung MK, Kim do H, Ahn YC, Choi JY, Kim EH, Son YI. (2016). Randomized Trial of Vitamin C/E Complex for Prevention of Radiation-Induced Xerostomia in Patients with Head and Neck Cancer. Otolaryngol Head Neck Surg, 155(3):423-30. doi: 10.1177/0194599816642418.
20. Zhao F, Wang YF, Song L, Jin JX, Zhang YQ, Gan HY, Yang KH. (2017). Synergistic Apoptotic Effect of D-Fraction From Grifola frondosa and Vitamin C on Hepatocellular Carcinoma SMMC-7721 Cells. Integr Cancer Ther, 16(2):205-214. doi: 10.1177/1534735416644674.
21. Parrow, N. L., Leshin, J. A., & Levine, M. (2013). Parenteral Ascorbate As a Cancer Therapeutic: A Reassessment Based on Pharmacokinetics. Antioxidants & Redox Signaling, 19(17), 2141–2156. http://doi.org/10.1089/ars.2013.5372
22. Cathcart R.F. (1981). Vitamin, C.; titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med. Hypotheses, 7:1359–1376. doi: 10.1016/0306-9877(81)90126-2.


